Isotherms
Tags
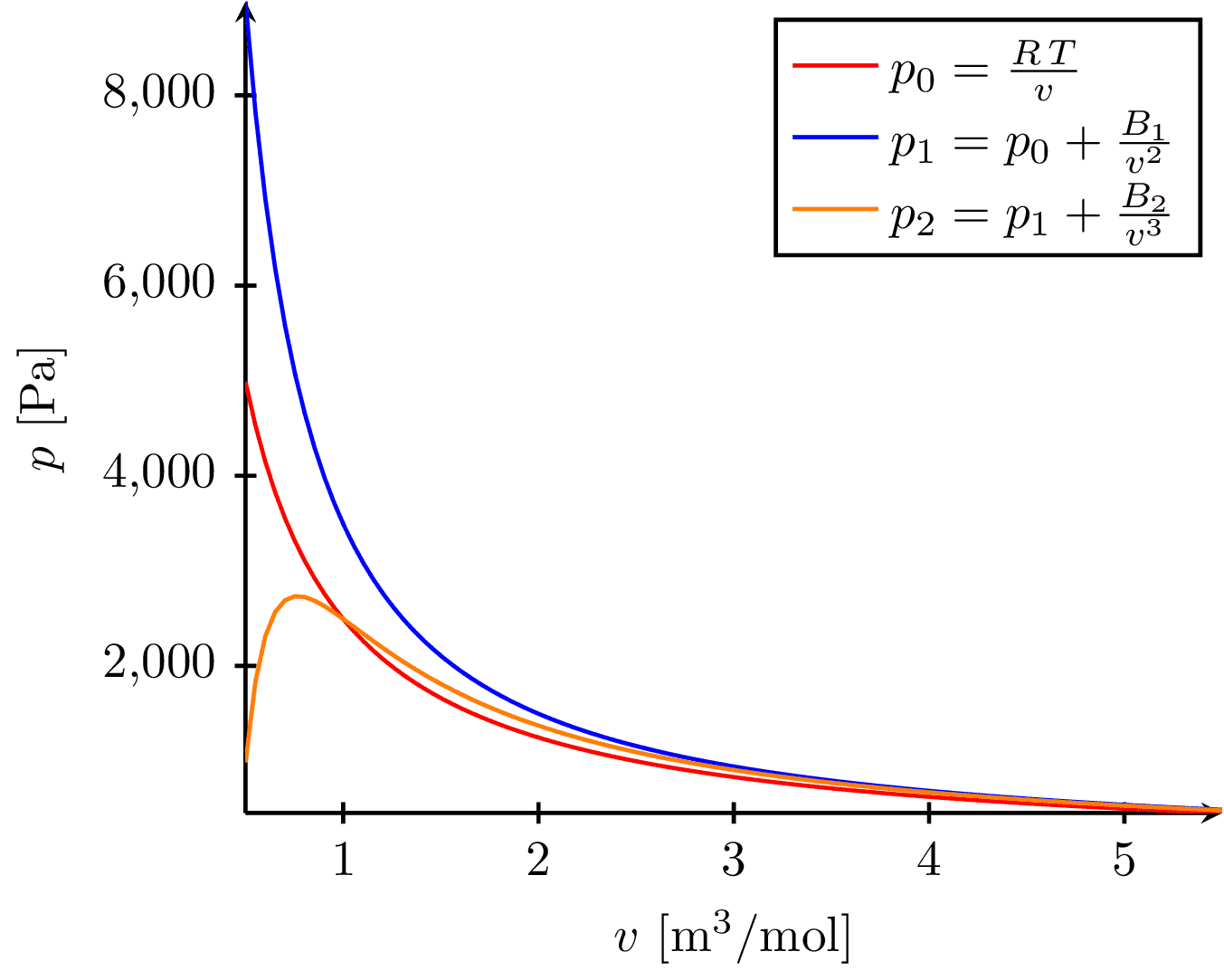
This image visualizes the behavior of gas isotherms (curves showing pressure-volume relationships at constant temperature), represented by three equations of state, as a function of volume. The equations include the ideal gas law, and two other modified laws accounting for molecular interactions. The x-axis represents the molar volume and the y-axis represents the pressure of the gas.
Edit
Download
Code
isotherms.tex (48 lines)
\documentclass{standalone}
\AtBeginDocument{\renewcommand{\AtBeginDocument}[1]{}}
\usepackage{siunitx}
\usepackage{pgfplots}
\pgfplotsset{compat=newest}
\begin{document}
\begin{tikzpicture}
\def\R{8.31}\def\T{300}
\def\a{10^(-3)}\def\b{10^(-5)}
% \def\Bone{(\b*\R*\T - \a)}
% \def\Btwo{(\b^2*\R*\T - \a*\b + \a^2/(2*\R*\T))}
\def\Bone{1000}
\def\Btwo{-1000}
\def\pzero{\R*\T/x}
\def\pone{\pzero + \Bone/x^2}
\def\ptwo{\pone + \Btwo/x^3}
\begin{axis}[
axis lines=left,
samples=100,
domain=0.5:5.5,
xlabel={$v$ [\si{\meter\cubed\per\mol]}},
ylabel={$p$ [\si{\pascal}]},
every tick/.style={thick},
thick]
% p_0
\addplot[color=red]{\pzero};
\addlegendentry[right]{$p_0 = \frac{R \, T}{v}$}
% p_1
\addplot[color=blue]{\pone};
\addlegendentry[right]{$p_1 = p_0 + \frac{B_1}{v^2}$}
% p_2
\addplot[color=orange]{\ptwo};
\addlegendentry[right]{$p_2 = p_1 + \frac{B_2}{v^3}$}
\end{axis}
\end{tikzpicture}
\end{document}